Oral iron
Oral iron is usually recommended as first-line therapy, as it is a cost-effective strategy to restore iron balance. Both ferrous and ferric forms are available, but only the ferrous form is recommended due to superior absorption. Ferrous sulfate is used most frequently, but ferrous gluconate and fumarate can also be used.1,2,3
Shortcomings of oral iron supplementation are: (1) Poor iron absorption (10–15%), (2) iron loss or iron requirements in excess of the absorbed dose, (3) low bioavailability, (4) poor tolerability, (5) leading to noncompliance. Iron absorption can be lowered by concomitant intake of iron absorption inhibitors like phosphates, phytates, and tanates in food and certain digestive disorders. Certain side effects like abdominal discomfort, nausea/vomiting, diarrhea, and/or constipation are directly related to the amount of elemental iron ingested.1,4,5,6
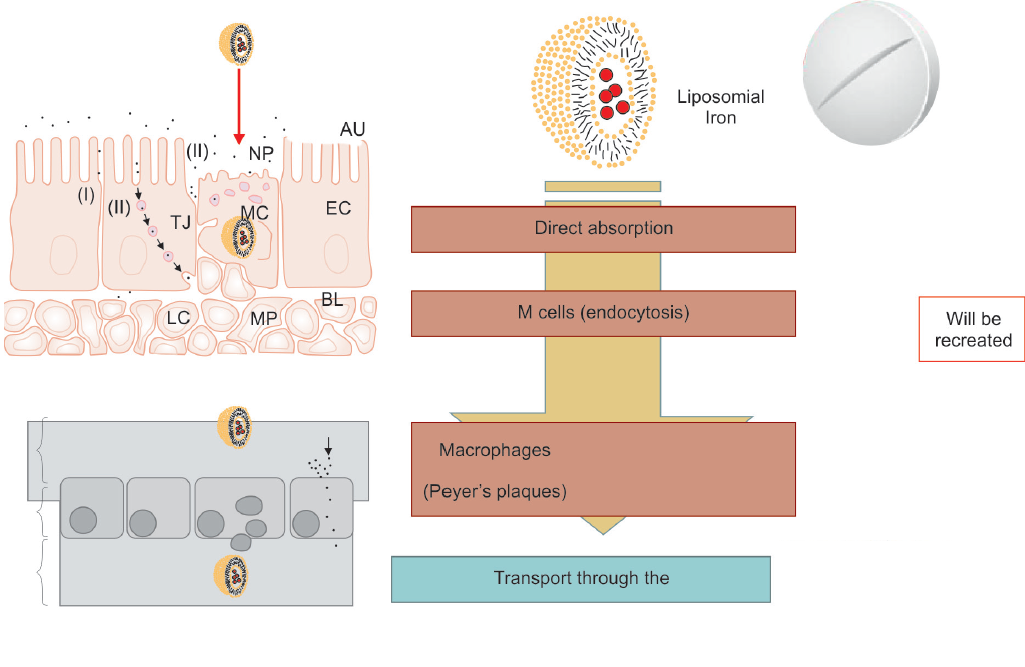
Fig. 2: Liposomal iron absorption
ORAL LIPOSOMAL IRON
Iron salts like ferrous pyrophosphate are covered with liposome, a spherical structure of a phospholipidic nature that is similar to those human cell membranes. This preparation crosses the gastric acid barrier and reaches the small intestine intact. In the intestine, the M cells due to their low lysozyme content integrally absorb liposomal iron without the need for specific transporters (Fig. 2).
Subsequently, the liposome is incorporated by endo-cytosis from macrophages and through the lymphatic stream it reaches, intact, the hepatocytes. The liposomal protection allows the iron to overcome the free gastric environment, preventing early degradation of the sub-stance and/or its inactivation and to be absorbed directly. This mechanism provides liposomal iron a greater avail-ability, reduces gastrointestinal side effects, and prevents iron instability in the gastrointestinal tract to be directly absorbed into the intestine and directly liberated into the liver.7-9
Consequently, this method of iron supplementation is associated with high gastrointestinal absorption, high bioavailability, and a low incidence of side effects.10 The absorption or bioavailability of liposomal pyrophosphate iron is 3.5 times greater than the free pyrophosphate iron, 2.7 times higher than iron sulfate, and 4.1 times higher compared with iron gluconate. In addition, the plasma concentration of liposomal iron was maximum after 2 hours from the assumption, which guarantees greater bioavailability of the element for all metabolic processes.7
CLINICAL USES
Chemotherapy-related Anemia
Anemia in cancer patients is common due to pathologic deficiency in the amount of oxygen-carrying Hb in red blood cells. Current treatment for chemotherapy- associated anemia often relies on the use of erythropoietic-stimulating agents. In one of the recent studies by Mafodda et al,11 a comparison between oral liposomal iron vs IV iron in anemic cancer patients receiving chemotherapy was made. Liposomal oral iron provided similar increase in Hb levels and Hb response, with higher tolerability without the risks or side effects of IV iron. Similarly, from baseline to study end, a mean increase in Hb levels of 2.2 gm/dL and improvement in quality of life (QoL) parameters was noted with liposomal iron in patients with chemotherapy-related anemia.12 Barni et al13 suggest that liposomal iron could be considered as a prophylactic measure to prevent transfusions/erythropoiesis-stimulating agents (ESAs) in cancer patients treated with chemotherapy and preexisting mild anemia.
Liposomal iron can be used as supportive therapy to reduce fatigue and improve QoL in patients with advanced prostate cancer and bone metastasis treated with monthly IV injections. It is also suggested that liposomal iron could be considered for its prophylactic use to prevent transfusion/ESAs in patients with preexisting mild anemia and to improve compliance at treatment with monthly IV injections of Radium-233 dichloride.14,15 In young advanced-stage Hodgkin lymphoma patients, supplementation of oral liposomal iron was well tolerated and maintained Hb above levels, requiring further supportive therapy.16
Iron Deficiency Anemia and Inflammatory Bowel Disease
A preliminary study showed that IDA and IBD patients on liposomal iron (10.5–12.4 gm/dL) had better increase in Hb levels as compared with patients on ferrous sulfate (10.8–11.7 gm/dL) or no iron supplement (11.3–11.9 gm/dL). An increase of Hb >2 gm/dL was more frequent in patients treated with liposomal iron than in patients with no iron supplement. Liposomal iron was well tolerated in both IDA and IBD patients.17 In a similar study, one-third of the IBD patients treated with liposomal iron were normalized in 12 weeks, with an average Hb increase of 11.1 to 11.8 gm/dL (p = 0.0023). Furthermore, the average rating in the questionnaire on QoL of IBD (CCVVEII-9) improved from 61.2 to 66.8 points on the final visit. An adherence of >90% and acceptance of >80% was noted. Therefore, liposomal iron can be helpful in those patients who do not tolerate classic prepared doses of oral iron.18 In refractory anemia, oral liposomal iron is found to safe, effective, and has demonstrated noninferiority over IV iron.19,20 Compared with conventional iron supplements like ferric ammonium citrate and heme iron, liposomal iron increased iron levels and HB concentrations so as to alleviate the anemia in murine models of sports anemia and anemia of inflammation.21 Liposomal iron was also effective in elderly patients, well tolerated, and produced a great improvement in the anemia condition without side effects, which helped in improving QoL.22 Liposomal iron was found to be more effective than iron sulfate in increasing Hb levels and to reduce inflammatory markers in correction of anemia of chronic inflammatory disease.23 The IBD patients are usually more frequently resistant to oral therapy and they show low compliance. Liposomal iron showed excellent compliance and treatment adherence.24
Patients with inactive/mildly active inflammatory bowel disease were screened for anemia in the interventional pilot study conducted from November 2016 to March 2018. Patients with mild anemia were treated with oral liposomal iron for 8 weeks. Conclusion: Treatment with oral liposomal iron is effective in improving mild iron deficiency anemia and quality of life, as well as in decreasing fatigue in patients with inactive or mildly active inflammatory bowel disease.25
Chronic Kidney Disease-related Anemia
In the preliminary study, 21 patients with CKD-related anemia were analyzed, 14 of whom were treated with oral liposomal iron and 7 with IV iron. The observed increase of Hb at 8 weeks compared with baseline was similar in both groups, but was significant in the liposomal group only.7 Data show that oral iron administration compared with the IV iron therapy showed a significant increase in terms of Hb concentration and transferrin saturation and a significant decrease regarding C-reactive protein values and weekly consumption of erythropoietin. In conclusion liposomal iron seems to be a valid alternative to IV iron therapy in CKD patients.26 A recent study by Pisani et al27 showed that oral liposomal iron was a safe and efficacious alternative to IV iron gluconate to correct IDA in nondialysis CKD patients. Oral liposomal iron was also effective in improving and or/maintaining Hb values in hemodialysis patients, normalizing ferritin values and significantly decreasing erythropoietin consumption.28 According to Griveas,29 oral liposomal iron seems to be a safe and efficacious alternative in managing CKD patients with anemia.15
Other researchers suggest that therapy with two capsules of liposomal iron daily could be an alternative therapy in hemodialysis patients with iron deficiency.30 Arenas et al31 showed that liposomal iron was effica-cious, well tolerated, and with an excellent therapeutic adherence treatment option for patients with nondialysis CKD. It is documented in one of the studies that addition of liposomal iron to the 12-week standard regimen with subcutaneous erythropoietin is effective in improving hematological parameters but also, more importantly, the QoL with no side effects and excellent tolerability in elderly anemic patients with no end-stage CKD.32 In anemic CKD patients at stages III to IV, supplementation with liposomal iron was associated with reduced activation of the inflammatory state, as assessed by reduction of erythrocyte sedimentation rate.33 It is demonstrated that liposomal iron was safe and efficacious in maintaining transferrin saturation levels in peritoneal dialysis patients. Although ferritin levels decreased, they remained within therapeutic range. No gastrointestinal adverse effects were reported.34
Celiac Disease
Patients with celiac disease (CD) frequently suffer from IDA and may benefit from iron supplementation. After a follow-up of 90 days, CD and IDA patients in both liposomal and sulfate groups showed an increase in Hb levels compared with baseline (+10.1 and +16.2% for liposomal and sulfate groups respectively), and a significant improvement in all iron parameters, with no statistical difference between the two groups. Therefore, liposomal iron can be effective in providing iron supplementation in difficult-to-treat populations.35
pregnant women with iron deficiency
This study aimed to determine the effects of liposomal iron pyrophosphate/ascorbic acid on clinical and psychological outcomes in pregnant women. Women at the 11th–13th weeks of gestation with iron deficiency anaemia assuming Sideremil™ from April 2018 to May 2019 were recruited. Haematochemical, obstetric, neonatal and psychological outcomes were investigated at the enrolment, 21–23 weeks of gestation, 30–32 weeks of gestation and after 6 weeks from childbirth. Results showed significant positive effects on haemoglobin, ferritin, sideremia and transferrin levels, compared to baseline data. A significant improvement of anxiety and depression levels was also observed. Regarding the quality of life, all the domains significantly improved, especially the Physical Role domain. Our results indicate that Sideremil™ may be a valid treatment for iron deficiency anaemia in pregnant women, since it significantly improves haematological and mental health outcomes. However, further studies are needed to confirm these results.36
Other Conditions
It was demonstrated that liposomal iron was effective in replenishing iron storage in cirrhotic patients and despite the use of a high dose, it is well tolerated.37 In diabetic patients with IDA supported with liposomal iron, the need for median lispro insulin was lower than that of he patients supported with IV sodium ferrigluconate.23 Similarly, liposomal iron was found to be safe and cost-effective in hepatitis C virus patients with type II diabetes and anemia due to esophageal or gastric bleeding.38 In diabetic patients with IDA supported with liposomal iron, the median lispro insulin need appears to be lower than that of the patients supported with IV sodium ferrigluconate. However, the study needs confirmation on a larger cohort of patients.38
Researchers assessed the effect of switching to oral liposomal iron in patients receiving IV iron supplementation after bariatric surgery, which currently requires parenteral iron therapy due to intolerance to existing oral products or therapeutic failure. Oral liposomal iron was found be an excellent alternative to IV iron for maintenance treatment in bariatric surgery patients with iron deficiency. It might help to reduce health care costs and improve the QoL of these patients.15,39 Liposomal iron was found to be more effective and well tolerated than iron sulfate for correction of anemia in systemic sclerosis patients who have both chronic inflammation and gastro-intestinal malabsorption issues.40 Liposomal iron therapy was found to be safe, well tolerated, and effective at least as a standard ferrous salt therapy in patients undergoing cytoreductive surgery with intraperitoneal hyperthermic chemotherapy.41
References
- Barragan-Ibaneza G, et al. Iron deficiency anaemia. Rev Med Hosp Gen Mex 2016 Apr-Jun; 79(2):88-97.
- Lopez A. et al. Iron deficiency anaemia. Lancet 2016 Feb;387(10021):907-916.
- Kaya Z. Iron deficiency anemia: current strategies for the diagnosis and management. Rev Health Care 2013;4(3):193-202.
- Liu K, et al. Iron deficiency anaemia: a review of diagnosis, investigation and management. Eur J Gastroenterol
Hepatol 2011 Feb;24(2):109-116.
- Fei, C. Iron deficiency anemia: a guide to oral iron supplements. [cited 2018 Jun 29]. Available from: https://www. clinicalcorrelations.org/?p=8405. Accessed on June 29, 2018
- Macdougall IC. Strategies for iron supplementation: oral versus intravenous. Kidney Int Suppl 1999 Mar;69(Suppl 69): S61-S66.
- Visciano B, et al. Il ferro liposomiale: una nuova proposta per il trattamento dell’anemia nell’insufficienza renale cronica. G Ital Nefrol 2013 Oct;30(5):1-9.
- Paz, GB. Efficacy and tolerability of Sucrosomial® iron supplementation in IBD patients with iron deficiency anemia and intolerance to iron oral salts. 3rd Mediterranean Multidisciplinary Course on Iron Anemia, Rome, Italy, April 17-18, 2015.
- Tarantino G, et al. Sucrosomial iron®: a new highly bioavailable oral iron supplement. Blood 2015 Dec; 126:4561.
- Locatelli F. et al. Iron therapy challenges for the treatment of nondialysis CKD patients. Clin J Am Soc Nephrol 2016 Jul;11(7):1269-1280.
- Mafodda A, et al. Oral sucrosomial iron versus intravenous iron in anemic cancer patients without iron deficiency receiving darbepoetin alfa: a pilot study. Support Care Cancer 2017 Sep;25(9):2779-2786.
- Prestifilippo A,et al.Safety and efficacy of oral liposomal iron supplemented in cancer patients with chemotherapy- related anemia receiving epoetin alfa: final data. ESMO Congress 2012, Viganello, Switzerland. 2012.
- Barni S, et al. Up-front Sucrosomial® iron supplementation in patients with preexisting G1 anemia before planned chemotherapy: a prospective observational study. Exp Rev Hematol 2016;9(Suppl 1):1-42.
- Monari F,et al. Oral Sucrosomial® iron (Sideral® Forte) supplementation in patients with advanced prostate cancer and bone metastasis treated with 223 radium dichloride. Exp Rev Hematol 2016;9(Suppl 1):1-42.
- Barni S. 4th mediterranean multidisciplinary course on iron anemia April 29th-30th 2016, Madrid, Spain. Exp Rev Hematol 2016 Sep;9(Suppl 1):1-42.
- Romano A, et al. Oral Sucrosomial® iron supplementation in patients affected by Hodgkin lymphoma with mild anemia before chemotherapy: an observational study. Exp Rev Hematol2016;9(S1):1-42.
- Indriolo A, et al. Comparison between liposomial iron and ferrous sulfate in patients with iron deficiency anemia and inflammatory bowel disease. A pilot controlled study. J Crohn’s Colitis 2014 Feb;8(Suppl 1):S289.
- Paz GB. Efficacy and tolerability of Sucrosomial® iron supplementation in IBD patients with iron deficiency anemia and intolerance to iron oral salts. Exp Rev Hematol 2016;9(Suppl 1): 1-42.
- Giordano G, et al. Intravenous iron support or oral liposomal iron support in patients with refractory anemia treated with Epo alpha. Leuk Res 2011 May;35(Suppl 1): S137.
- Giordano G, et al Biosimilar epoetin α is as effective as originator epoetin-α plus liposomal iron (Sideral®), vitamin B12 and folates in patients with refractory anemia: a retrospective real-life approach. Mol Clin Oncol 2015 Jul;3(4):781-784.
- Yu PP,et al. Iron liposome: a more effective iron supplement for sports anemia and anemia of inflammation. J Pharma Care Health Sys 2015 Dec; S4:002.
- Nasutia A, et al. Oral Sucrosomial® iron (Sideral® Forte) is effective and well tolerated in elderly patients affected by iron deficiency anemia of various origins. Exp Rev Hematol 2016;9(Suppl 1):1-42.
- Giordano G, et al. Sucrosomial iron better than iron sulfate in corrections of anemia of chronic inflammatory disease of young women. Italian Society of Hematology Congress. 2013.
- Romano M. Sucrosomial® iron is effective in correcting inflammatory bowel disease anemia and is more tolerable than sulfate iron. Exp Rev Hematol 2016;9(Suppl 1):1-42.
- de Alvarenga Antunes, C.V, et al.Treatment of iron deficiency anemia with liposomal iron in inflammatory bowel disease: efficacy and impact on quality of life. Int J Clin Pharm 42, 895–902 (2020).
- Ralli C, et al. Comparative study between Sideral forte and intravenous iron in chronic kidney disease. Exp Rev Hematol 2016;9(Suppl 1):1-42.
- Pisani A, et al. Effect of oral liposomal iron versus intravenous iron for treatment of iron deficiency anaemia in CKD patients: a randomized trial. Nephrol Dial Transplant 2015 Apr;30(4):645-652.
- Pistoni, G, et al. Effectiveness of Sucrosomial® Iron (Sideral® Forte) in dialysis patients in therapy with intravenous iron and erythropoietin (EPO). 57th Congress of Italian Society of Nephrology, Rome. 2016.
- Griveas I, et al. Effect of oral Sucrosomial® Iron in CKD patients with anemia. Poster Discussion. Exp Rev Hematol 2016;9(Suppl 1): 1-42.
- Brunati C, et al. Sucrosomial® Iron therapy in dialysis. Exp Rev Hematol 2016;9(Suppl 1):1-42.
- Arenas MD, et al. Efficacy, tolerance, and adherence to treatment with Sucrosomial® Iron in patients with chronic kidney disease stages 3-4 and iron deficiency. Exp Rev Hematol 2016;9(Suppl 1):1-42.
- Equitani F. Erythropoietin (EPO) plus oral Sucrosomial® Iron versus EPO alone for the treatment of severe anemia in no end-stage chronic kidney disease. Exp Rev Hemat 2016;9(Suppl 1):1-42.
- Altieri D, et al. Effects of oral Sucrosomial® Iron (Sideral® Forte) on inflammatory markers and endothelial dysfunction in CKD patients: preliminary data. Exp Rev Hematol 2016;9(Suppl 1):1-42.
- Duarte V. et al. Oral Sucrosomial® Iron in peritoneal dialysis patients. Exp Rev Hematol 2016; 9(Suppl 1):1-42.
- Elli L, et al. Sucrosomial iron supplementation in anemic patients with celiac disease not tolerating oral ferrous sulfate: a prospective study. Nutrients 2018 Mar;10(3): E330.
- Vitale SG, et al. Liposomal ferric pyrophosphate and ascorbic acid supplementation in pregnant women with iron deficiency anaemia: haematochemical, obstetric, neonatal and psychological outcomes in a prospective observational study July 2021International Journal of Food Sciences and Nutrition 73(10):1-9
- Vallerioa P, et al. Sucrosomial® Iron and aortic stiffness in cirrhotic patients. Exp Rev Hematol 2016;9(Suppl 1): 1-42.
- Giodarno G, et al. Reduced insulin need in patients with type 2 diabetes mellitus(T2DM) with iron deficiency anemia treated with Sucrosomial® iron versus intravenous sodium ferrigluconate. Multicentric prospective study. Exp Rev Hematol 2016 Jun;9(Suppl 1):1-42.
- Ciudin A, et al. Response to oral sucrosomial iron supplementation in patients undergoing bariatric surgery. The BARI-FER study. Endocrinol Diabetes Nutr 2018 Jan;65(1): 17-20.
- Parisi S, et al. Efficacy of Sucrosomial® iron (Sideral® Forte) in the treatment of anemia in patients affected by systemic sclerosis. Exp Rev Hematol 2016;9(Suppl 1):1-42.
- Barragans M, et al. Sucrosomial® iron versus ferrous sulfate for anemia in patients undergoing peritoneal carcinomatosis with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Exp Rev Hematol 2016;9(Suppl 1):1-42.